
Scaling Incrementally
From a little joint venture to a $20B Organization, the agile journey of one of the largest health care product organizations on the planet tested everything we knew, or thought we knew, about agile at scale. We highlight some key aspects of that journey...

Pete Behrens and Ross Hughes share their experiences and perspectives of the GE Healthcare Agile Transformation from 2008 to 2014. Pete was brought in as an external agile coach. Ross was the project manager turned principle internal change agent for the agile transition once it was brought into the GE Healthcare enterprise.
Organizational Challenges
Over many decades of regulations, many of the GE Healthcare product lines developed rigorous safety and reliability procedures due to their industry expectations and fears of mistakes. Further, financial controls limited changes in the product cycle due to some US-based financial incentives for software capitalization. While well intended, these structural and procedural decisions handcuffed the organization’s innovation and ability to deliver in time to meet market demands.
Due to increased competitive pressures in the marketplace, leadership within GE Healthcare desired a faster innovation cycle with a focus on shortening the discovery and delivery cycle to more quickly evaluate new ideas and projects.
Incremental Agility
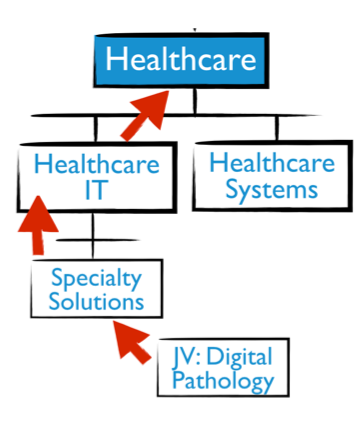 GE Healthcare did not trust agile enough to try it out in their organization. So to enable a new way of working, they allowed one of their joint ventures to experiment. The joint venture in Digital Pathology was unhampered with the policies of broader organization. Leaders there were able to experiment with agile approaches in a safe-to-experiment culture, separated from the low-risk tolerant main culture.
GE Healthcare did not trust agile enough to try it out in their organization. So to enable a new way of working, they allowed one of their joint ventures to experiment. The joint venture in Digital Pathology was unhampered with the policies of broader organization. Leaders there were able to experiment with agile approaches in a safe-to-experiment culture, separated from the low-risk tolerant main culture.
Once these early experiments proved valuable, leaders were able to introduce them into the broader organization. These next experiments then began to challenge internal structures, policies and metrics to improve their overall agile ways of working. Over many years, the entirety of the healthcare organization became exposed to and is leveraging agile approaches.
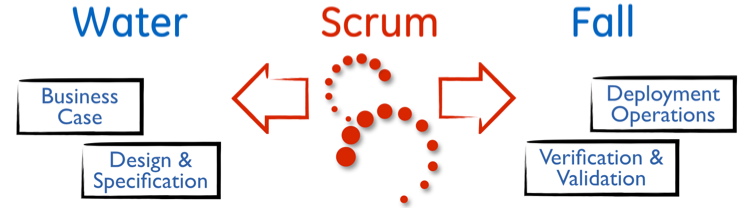
As a culture requiring saftey, reliability, repeatability, and stability, changing the entire product lifecycle was out of the question. So we found out what was possible, or as Ken Schwaber likes to call it – the art of the possible. In the case of GE Healthcare product lines, this meant that we did NOT touch the initial business case and product definition phases. Further, we did NOT touch the final Verification and Validation (V&V) phases. Thus, we had retained key “waterfall” elements and simply inserted Scrum in the middle. Some companies call this “agile”. We called it “Water Scrum Fall”. This allowed the benefit of learning Scrum and trusting Scrum. Because through Scrum we began to realize we required less time in final V&V phase due to more testing in the Scrum Framework. Further, we realized that we could be confident in building the right thing even with less formal product definition documents. Scrum was spreading.
Key Organizational Experiments
- Increased release frequency using a Quarterly Release Train to align all teams on a common sprint and delivery cycle and offset financial and regulatory risk.
- Enhanced automated testing and moved testing from the final verification stage into the sprint development and test cycle.
- Shifted leadership expectations from plan commitments to forecasts to foster increased creativity and speed.
- Introduced a Lean Startup approach to test assumptions earlier in the design cycle.
- Created an Agile Team/Program Health Barometer to measure agile maturity.
- Incorporated the Scaled Agile Framework (SAFe) to drive consistency in approach.
Agile Ways of Working Results
- 75% improvement in time to market from 1-2 years to every 3-6 months through a reduction in product complexity.
- 50% increase in feature adaptability based on customer feedback through elimination of financial capitalization constraints.
- 40% reduction in defects found in verification phase and 30% reduction in testing time.
- Shrank a typical 6-month Verification and Validation (V&V) Test Cycle from 6-months to 1-month or less.
